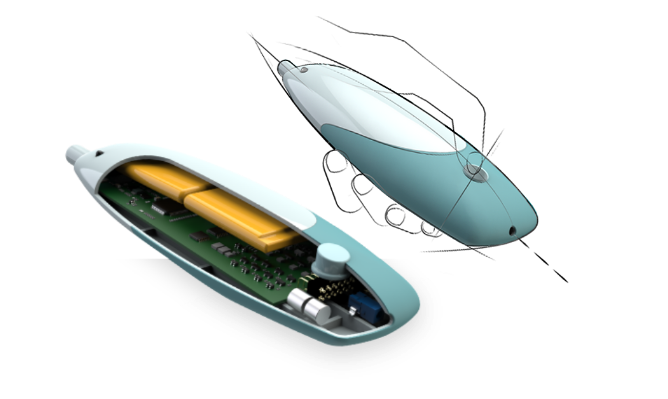
Product Design
- In-house Product Design team
- Requirements gathering including product and user requirement specification
- Medical Device design with human factors and usability in mind
- Proof of Concept development
- Rapid prototype and test using in-house 3D printing lab
- Aesthetic plastic enclosure design
- Industrial design with integration of mechanical and electronic systems using Solidworks and Inventor
- Demonstrator level prototype development
- Functional prototype development
- Fabric based wearable devices
- Adhesive based wearable medical devices
- Complex Electro-mechanical systems
- Design History file and design documentation to ISO 13485 and FDA technical file standard

Embedded Electronic & Firmware Design
- In-house embedded electronic and firmware development team
- Analog and digital electronic design
- Sensor based smart systems design
- Wearable IOT device development
- Communications development experience in: Bluetooth, NBIOT, Wifi, Lora and Cellular
- System requirements specification
- Electronic architecture development and key component selection
- Schematic development, PCB layout, prototype and test
- Firmware development to EN 62304
- Electronic proof of concept, functional prototype and Design For Manufacture
- Manufacturing release level Electronics and Firmware design
- Design History file and design documentation to ISO 13485 and FDA technical file standard
Mechanical Design
- In-house Mechanical Design team
- Plastics and metal part design expertise
- Component and material selection
- Full system mechanical design using Solidworks and Inventor.
- Mechanical solution concept development
- Custom Mechanism development
- Design for Biocompatibility
- Design for harsh environmental conditions
- Integration of complex electro-mechanical systems such as pumps, vacuums & fluidic systems in medical devices
- Design for injection moulding and tooling launch in manufacturing
- Support of Design transfer to manufacturing
- Complex Mechanical System Design
- Design History File and Design documentation to ISO 13485 and FDA technical file standard
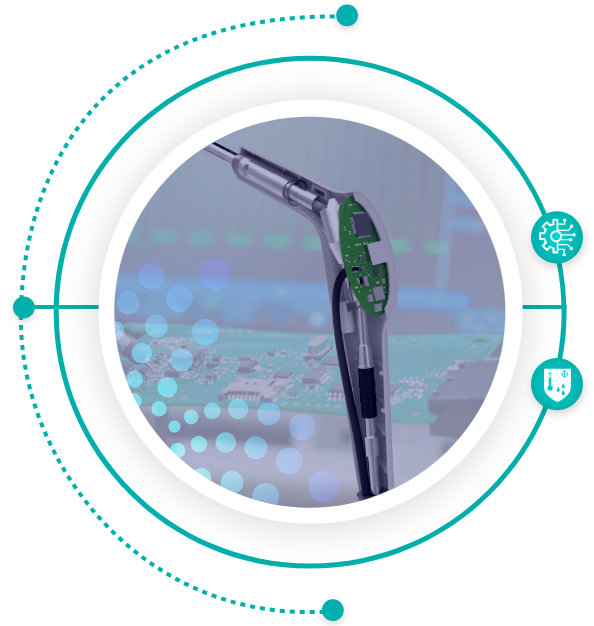
Electro-Mechanical Integration
- In-house Electro-Mechanical Integration experts
- Complex integration of electronics to mechanical systems
- Design for environmental conditions
- Integrated design concept for overall system
Design for Manufacture
- Mechanical system Design For Manufacture (DFM)
- Electronic System Design For Manufacture (DFM)
- Manufacturing process development for new product
- Key component supply chain development
- Design and implementation of manufacturing and supply chain solution for new product
- Full V+V support for new product technical file
Specialised Medical Device Design That Supports Our Customers’
Product Development Journey
At each phase, we combine the optimal medical device design team, capabilities and technologies with high levels of client collaboration to deliver a seamless experience from project kick-off to product launch.

Certifications and Regulatory Compliance
Gentian Health is certified to the medical device standard ISO 13485 for both Design and Manufacture of electronic products. Our team has a proven track record of designing for and attaining the following certifications across a range of products and projects CE, FCC, FDA and UL.
Gentian Health’s regulatory expertise and focus on quality ensures your device will meet the highest industry standards and global compliance requirements.