Markets
Medical Devices
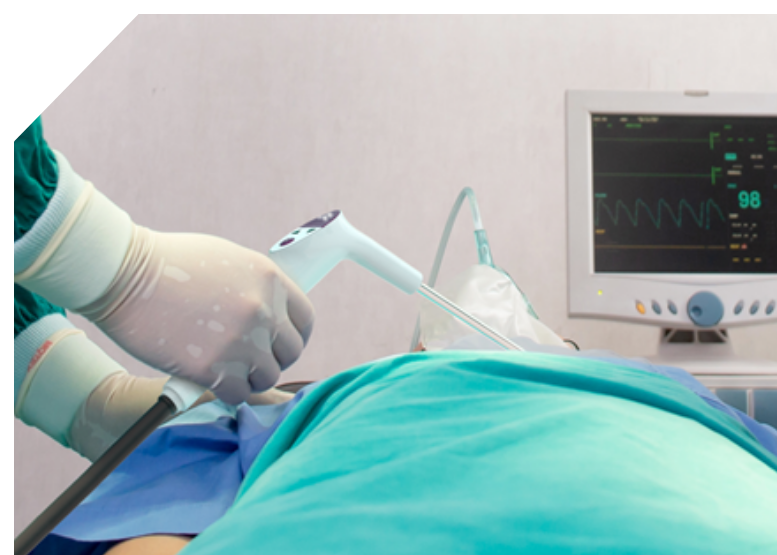
Gentian Health specialises in medical equipment design and manufacture of Class II medical devices and surgical instruments which involve the integration of both electronic and mechanical parts.
Over the years, our ISO13485 certified design team has worked with clients to develop and manufacture a range of both invasive and minimally invasive surgical and medical devices which are used in critical procedures.

Our Approach
Our team is experienced in medical equipment design and manufacturing. We have collaborated with clients on single-use devices which involve both electronic and mechanical design elements and must be built in a clean room and require sterilization. Starting with usability and human factors design Gentian Health’s cross-functional team then completes the detailed industrial design, mechanical design, embedded electronic design and full integration of electronic and mechanical systems.
Design transfer to manufacturing is a critical element of Gentian Health’s process and our team includes manufacturing engineers along with regulatory specialists who help migrate the product from the design team through to manufacturing in our onsite clean room.
Design for manufacture and early-stage manufacturing process design are layered in at each stage of the product development lifecycle ensuring that the first batch built for clinical trial is representative of full-scale manufacturing and is suitable for all clinical and regulatory activity.
We are experts in medical equipment design and manufacturing and every project is carried out with the highest quality standards. Gentian provides full Design V+V and Design history file along with process validation documentation and device history records for Clinical trial and follow on manufacturing batches.